Support:Documents:COMKAT Examples
Example 1: Estimate Input Parameters, Rate Constants Known -> Examples: Estimate Input Parameters, Rate Constants Known
Example 2: Simplified reference tissue method -> Examples: Simplified reference tissue method
Example 3: Glucose Minimal Model -> Examples: Glucose Minimal Model
Example 4: Enzyme-substrate (Michaelis-Menten) kinetics -> Examples: Enzyme-substrate (Michaelis-Menten) kinetics
Example 1: Estimate Input Parameters, Rate Constants Known
Simplified reference tissue method
Glucose minimal model
This example shows a COMKAT implementation of the glucose minimal model (GMM) which describes the interaction between glucose and insulin in vivo. One model compartment represents interstitial insulin concentration which is denoted by X. Another model compartment represents plasma glucose concentration which is denoted by G. This model was presented in Bergman RN, Ider YZ, Bowden CR, Cobelli C. "Quantitative estimation of insulin sensitivity," Am. J. Physiol., vol. 236, no. 6, pp. E667-E677, June 1979.
Background
Of particular note is that this model has an unusual nonlinear kinetic rule that is different from the nonlinear receptor-ligand and enzyme-substrate kinetic rules. Implementing this GMM in COMKAT required definition of a custom kinetic rule as this example demonstrates. This example also demonstrates COMKAT's ability to solve sensitivity functions (and hence estimate the value of) a parameter which is an initial condition to the differential equations.
Model Equations
dX/dt = -p2 X + p3(I - Ib)
dG/dt = -GX + p1(Gb - G)
with initial conditions X = 0, G = p4 at time zero.
X is interstitial insulin concentration. G is plasma glucose concentration. I is plasma insulin concentration. Ib and Gb are basal insulin and glucose concentrations. p1 to p4 are model mathematical parameters. They are related to the physiologically meaningful parameters as follows
- Glucose effectiveness, SG = p1, a measure of the fractional ability of glucose to enhance its own clearance independent of insulin
- Insulin sensitivity, SI = p3/p2, a measure of the dependence of fractional glucose disposal on changes of plasma insulin
- Initial glucose concentration, G(0) = p4
COMKAT Implementation
To implement the unusual non-linear kinetics present for glucose in COMKAT, the glucose state equation is rearranged to obtain
dG/dt = -G(X + p1) + p1Gb
and the expression (X + p1) is considered as a rate "constant".
Otherwise, the COMKAT implementation is straighforward and the diagram is depicted below.
Model Diagram
Since COMKAT requires a closed system, two junk or sink compartments were created (J1 and J2) so that the efflux from X and G would have a destination.
The complete example is given in comkat\examples\comkatGMM.m.
As the glucose minimal model is used to analze blood sample data (and not an image-based data set) for which people usually assume blood samples represent instantaneous (and not the time-averaged) concentration, this example only considers compartment concentrations (and not the output equations). Accordingly, the plots here are based on solutions to the compartment concentrations which are placed in comp when the model is solved using this command:
[out, outIndex, comp, compIndex] = solve(cm);
Plots of G and X versus time are shown here:
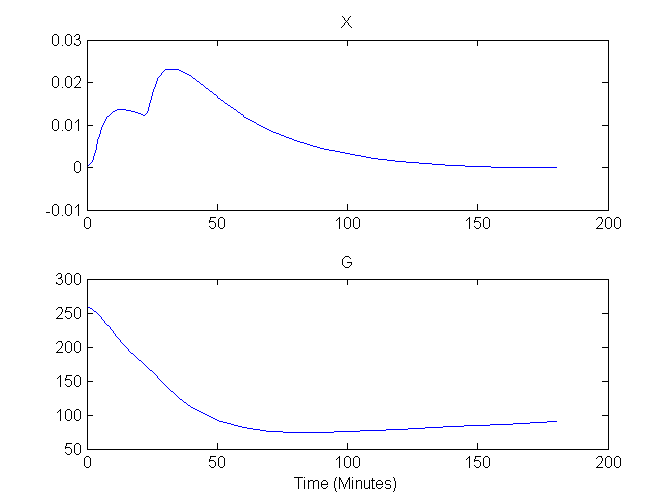
Compartment Concentrations and plots of compartment sensitivity functions, derivatives of X and G with respect to the model parameters, are shown below
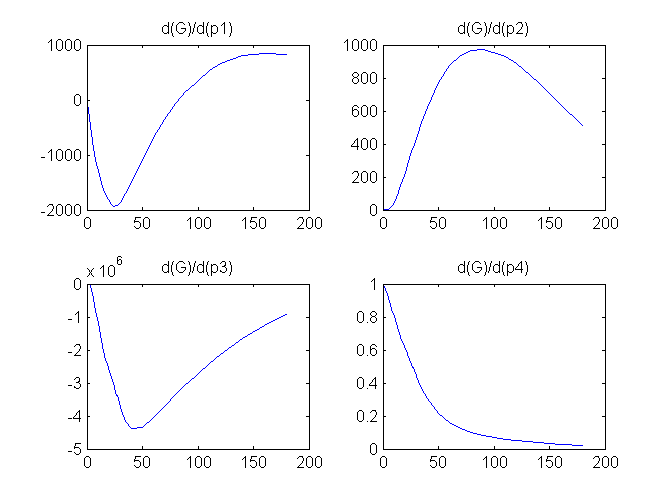
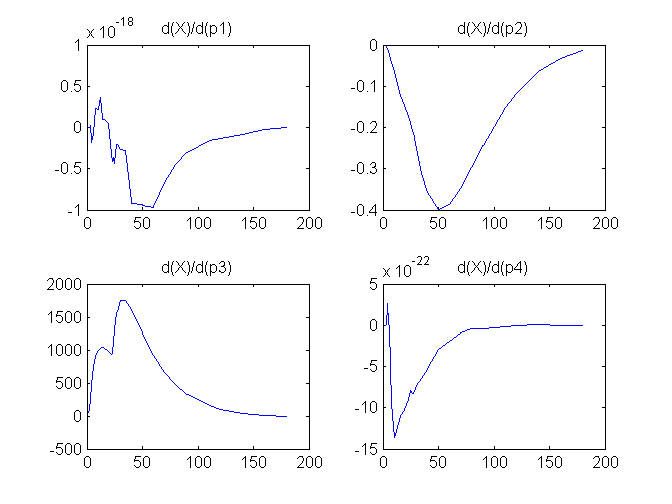
Notice that the plots of the sensitivity functions suggest variable X is independent of parameters p1 and p4 as d(X)/d(p1) and d(X)/d(p4) are zero (within the numerical precision). This is expected because a) the state equation for X has neither p1 nor p4 terms and b) G, which appears on the right hand side of the state equation for X, is independent of p1 and p4.
NOTE: GMM is also implemented as part of the validation suite wherein solutions to a COMKAT and an independent implementation are compared.
Enzyme-substrate (Michaelis-Menten) kinetics
This example demonstrates COMKAT implementation of enzyme substrate (Michaelis Menten) kinetics. This is a type of kinetic rule in which reaction velocity is saturable. As such, this example demonstrates use of a user-specified custom kinetic rule.
Background
Enzymes act as catalysts speeding up reactions wherein a substrate is converted to product without the enzymes themselves being consumed in the process. When the amount of enzyme is much less than the amount of substrate, the effective rate constant k is not actually a constant but rather depends on the substrate concentration
k = Vmax / (Km + [S])
This relationship is described and derived here and requires a couple of approximations.
A COMKAT implementaion of a model that includes such kinetics is given below. The complete model that avoids the two approximations could also be implemented in COMKAT and will be left as an exercise for the student ;-).
COMKAT Implementation
Model figure
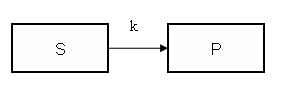
This example demonstrates how to implement in COMKAT a model that incorporates the Enzyme-Substrate kinetics using a rate "constant" with a value that depends on substrate concentration. In the interest of simplicity, this example only includes only two compartments and one link. Of course more complicated models could be constructed, yet this example is sufficent to demonstrate the concept.
Step 1 To set this up in MATLAB for simulation, create the following function that defines the rate "constant" for this kinetic rule:
function varargout = kinMM(t, c, ncomp, nsens, pName, pValue, pSensIdx, cm, xtra)
% Michaelis Menten kinetics
% flux = C * Vmax/(Km + C) so that effective
% rate constant k = Vmax/(Km + C)
VmName = pName{1};
KmName = pName{2};
VmValue = pValue{1};
KmValue = pValue{2};
VmSensIndex = pSensIdx{1};
KmSensIndex = pSensIdx{2};
substrateCompartmentIndex = xtra{1};
if (nargout > 0), % return effective rate constant
varargout{1} = VmValue / (KmValue + c(substrateCompartmentIndex));
if (nargout > 1), % return dk/dc
dkdc = zeros([1 ncomp]);
dkdc(substrateCompartmentIndex) = VmValue / (KmValue + c(substrateCompartmentIndex)).^2;
varargout{2} = dkdc;
if (nargout > 2), % return dk/dp
dkdp = zeros([1 nsens]);
dkdp(VmSensIndex) = 1 / (KmValue + c(substrateCompartmentIndex));
dkdp(KmSensIndex) = VmValue / (KmValue + c(substrateCompartmentIndex)).^2;
varargout{3} = dkdp; % dkdp
end
end
end;
return;
As for all custom kinetic rules, this function must adhere to the call signature with 8 (or optionally 9) input arguments whether or not all inputs are used. The inputs are
t time
c Vector of compartment concentrations
ncomp Number of compartments in the model
nsens Number of parameters for which sensitivity functions are requested (e.g. the number of parameters being estimated_
pName Cell array of character strings. E.g. pName{1} = 'Vm', pName{2} = 'Km'
pValue Cell array with pValue{i} being the value of pName{i}
pSensIdx Cell array containing indicies (locations) of pName{i} in the sensitivityName [addSensitivity()] parameters.
cm The compartmentModel object
xtra Any extra information that the user would like to be passed to the function [this input is optional]
The kinMM function is bit complicated because it was written to be general. The compartments that are the source and destination of the link are not hardcoded but rather determined based on the value of the input arguments. Likewise, the names of the parameters that are used to identify what are traditionally described as Km and Vmax are also be specified. Not only does this allow a user call the parameter whatever suits his or her fancy (e.g. the Km value could be specified by a parameter called 'Fred'), it also facilitates models with more than one link that is described by enzyme-substrate kinetics. Writing the function to be general in this fashion obviates having to write a separate kinetic rule function for each model. (The newest verion of the function above is available in comkat\examples\kinMM.m).
One thing to notice about the function above is that the line
varargout{1} = VmValue / (KmValue + c(substrateCompartmentIndex));
corresponds to the equation given in Background.The substrate concentration [S] is taken as the concentration in the source compartment.
Another thing to notice is that the function should return 0, 1 or more values depending nargout. The first return value would be the value of the rate "constant". The second return value would be the derivaive of the rate constant with respect to the concentration vector. The third return value would be the derivative of the rate constant with respect to the sensitivity parameters. These latter two are used in calculating the derivative of model output with respective to sensitivity parameters which is important in data fitting.
Step 2 Create the model.
conc = 10; % initial substrate concentration
cm = compartmentModel;
cm = addCompartment(cm, 'Substrate');
cm = addCompartment(cm, 'Product');
cm = addParameter(cm, 'Km', 10);
cm = addParameter(cm, 'Vm', 0.5);
cm = addParameter(cm, 'dk', 0); % specific activity
cm = addParameter(cm, 'sa', 1); % decay constant
% define the time
tmin = 0;
tmax = 120;
t = [0:1:tmax]';
scanTime = [t t+0.01];
cm = set(cm, 'ScanTime', scanTime);
% details needed for MM kinetics link
substrateCompartmentIndex = { 1 };
pName = {'Vm', 'Km'}; % Vm and Vm parameter names
cm = addLink(cm, 'KSpecial', 'Substrate', 'Product', 'Kmm', 'kinMM', pName, substrateCompartmentIndex);
% set initial concentration and TIME
IC.time = 0;
IC.compartment = [conc; 0]; % all substrate, no product
cm = set(cm, 'InitialConditions', IC);
% run the model
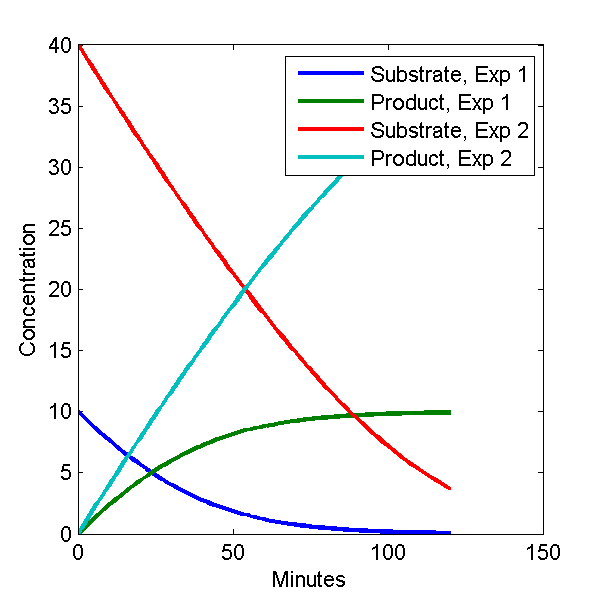
[cAvg, cAvgIdx, c1, cIdx] = solve(cm);
% repeat experiment with more substrate
IC.compartment = [4*conc; 0]; % all substrate, no product
cm = set(cm, 'InitialConditions', IC);
[cAvg, cAvgIdx, c2, cIdx] = solve(cm);
% plot results
plot(c1(:,1), c1(:,[2 3]), c2(:,1), c2(:,[2 3]));
legend('Substrate, Exp 1', 'Product, Exp 1', 'Substrate, Exp 2', 'Product, Exp 2')
The plot looks like this.
Model Output Plot
A slightly more elaborate version of this example can be found in comkat\examples\tutorialMichaelisMenten.m